Centrifuge Solutions for Therapeutic Monoclonal Antibody Research
By Florian Bundis, PhD, and Kate Meola, Eppendorf North America
Antibodies dominate today’s biopharmaceutical market, with drugs like Humira (AbbVie) and Avastin (Roche [Genentech]) among the top-selling drugs worldwide.1 The use of monoclonal antibodies (mAbs) has proven to be effective for treating various types of cancer and autoimmune diseases.
The development and production of mAbs using hybridoma or hybrid cells produced by fusing an antibody-producing lymphocyte with a tumor cell is now widely established and has been extended into SARS-CoV-2 vaccine development research. But rising time and cost pressures along with the demand for higher throughput make the development of therapeutic antibodies challenging.
"Rising time and cost pressures along with the demand for higher throughput can make the development of therapeutic antibodies challenging."
Optimizing Results
Factors that affect reproducibility, efficiency, and sample protection must be evaluated and new alternative solutions must be explored to optimize results and meet the high demand. Ergonomically designed equipment can help reduce hands-on time, protect staff from incurring work-associated injuries, and improve overall productivity and performance.
Multiple centrifugation steps occur during the mAb drug discovery process, each with unique speed, vessel, and temperature requirements. Based on in-depth protocol evaluations and customer interviews within the mAb drug discovery community, Eppendorf has developed seven centrifugation options that include the combination of an optimal centrifuge, rotor, and vessel adapters for each stage of the drug discovery workflow.
Eppendorf’s seven centrifugation solutions for therapeutic mAb research include:
- Molecular biology, ideal for spin column-based MiniPrep kits for nucleic acid purification, gel extraction, and PCR clean-up
- High speed, ideal for lysate clarification at 20,000 x g in nucleic acid purification protocols for target antigen production and antibody humanization
- Big volume harvesting, ideal for harvesting cells from large volume suspensions to produce antibodies in cell lines and antigens in bacteria
- Versatility, ideal for purifying peripheral blood mononuclear cells (PBMC), FACS/Flow cytometry, and hybridoma technology in multiple vessels
- High-throughput screening, ideal for identifying antibody hits
- Low-throughput screening, ideal for screening assays for selection and characterization of antibody leads
- Classic cell culture, ideal for cultivation and selection of stable cell lines
For example, high-throughput screening to identify antibody “hits” in the supernatants of hybridoma clone cultures is usually performed using microtiter plates (MTPs). Although various methods can be used, all include centrifuging temperature-sensitive samples in MTPs multiple times.
Eppendorf’s refrigerated benchtop centrifuge 5920 R, combined with swinging bucket rotor S-4x1000 and plate/tube buckets, can accommodate up to 28 MTPs per run. The Dynamic Compressor Control, a dynamic cooling technology, provides optimal sample protection. Continuous cooling ensures a constant temperature after the run is complete, and its soft-touch lid closure and quiet operation contribute to a comfortable working environment.
Eppendorf knows that your results are critical. Whether you work with therapeutic antibodies or vaccines against SARS-CoV-2, Eppendorf centrifuges can help you overcome your challenges and support your demand for sample protection, efficiency, and reproducibility.
- Genetic Engineering & Biotechnology News, April 2019. https://www.genengnews.com/wp-content/uploads/2019/03/GEN_Apr19_AList_Table.jpg

Above: High-throughput screening centrifuge solution
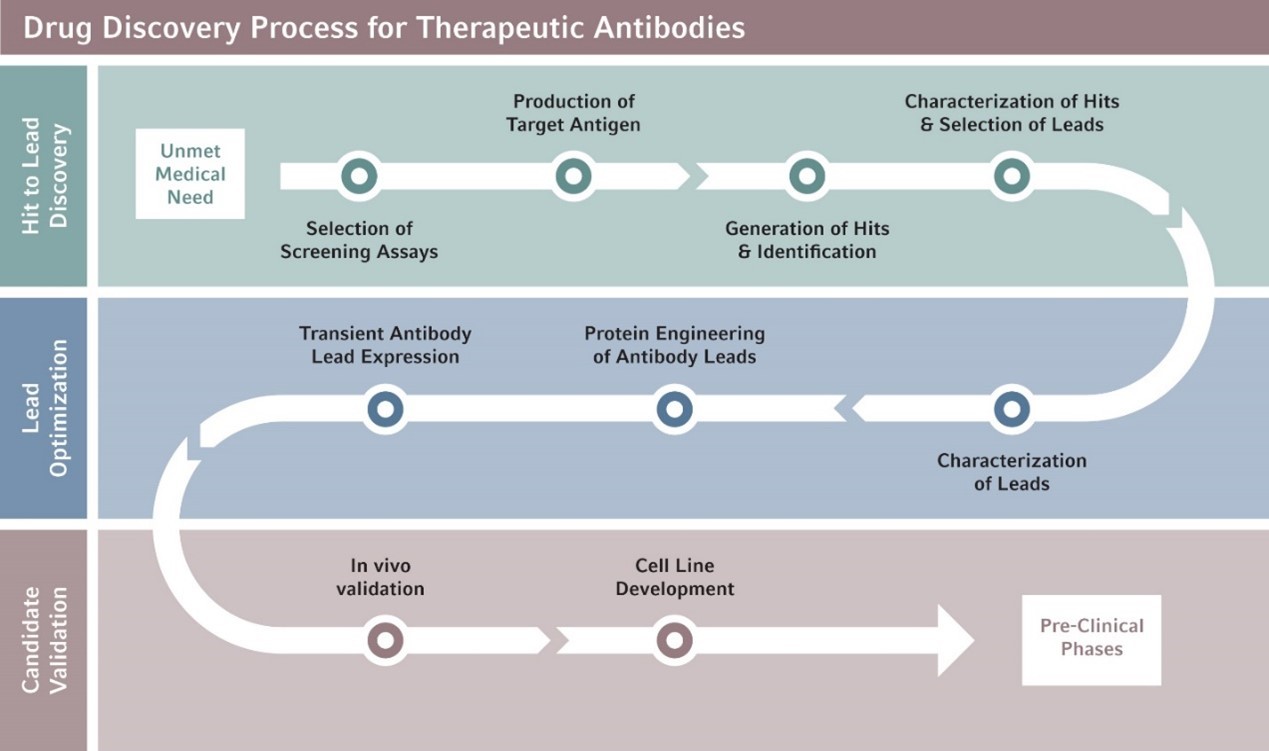
Above: Workflow of the drug discovery process for therapeutic mAbs

Content provided by:
